Daily Dose of Dinos
Absolute Dating
SUSD5 version of the Absolute Dating Lab
Purchase this Absolute Dating Activity at Teachers Pay Teachers for $1.00 Cents.
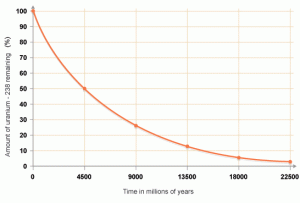
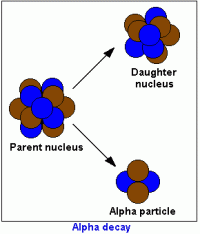
Absolute dating is used to determine the exact date of rocks by using their atoms. Scientists need to understand radioactive decay in order to absolute date. Atoms decay by losing a neutron and gaining a proton. When the number of protons in an atom is changed we call this radioactive decay and through this process, new elements are formed. When the element Uranium 238 decays, it ends up as lead-206 which isn't radioactive and therefore won't decay anymore. We call the uranium-238 the parent atom and the lead-206 the daughter atom.
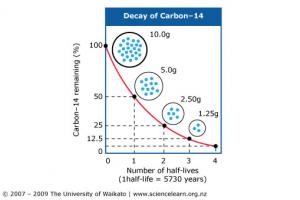
Understanding how long it takes for one element to become another element through radioactive decay is essential to absolute dating. A half-life is the time it takes for half of the atoms in an isotope to decay. One half-life of carbon-14 takes 5730 years. Two half-lives are 11,460 years and three half-lives are 17,190 years. Given enough half-lives, there eventually isn't enough carbon that remains. Using carbon to date rocks is called radiocarbon dating and is only good up to about 50,000 years because eventually there isn't enough carbon left to use.
By measuring the amounts of parent and daughter materials in rock and by understanding the half-life of the parent we can calculate the age of rocks. Geologists need to use the right isotopes to determine ages because for example fossils 1 billion years old don't have carbon left so we have to use another isotope like uranium-238 which has a half-life of 4.5 billion years. This radiometric dating technique is used when dating igneous and metamorphic rock, but not sedimentary rock. Why do you think that is? Using uranium-238 scientists have discovered that the oldest rock found is 3.96 billion years old. Scientists used that data to estimate the age of the Earth to be around 4.6 billion years old.
Scientists have also used an element called Zircon to date the age of the Earth. The video below describes how Zircon helps give an age to the Earth.
Uniformitarianism
Uniformitarianism states that the processes occurring today are similar to those that occurred in the past. So if Uranium-238 is decaying at a specific rate, then scientists believe that it decayed at the same rate throughout the ages. This concept was brought up by a fellow named James Hutton who felt that the Earth was much older than a few thousand years. He observed rocks and noticed that changes happen very, very slowly. Hutton also felt that the observable Earth processes at work today on the Earth, as well as in it, would be similar to the processes in the past. Another scientist named Lyell took it a little further by dating some of the Earth's processes.